
Eudamed is the European Database for Medical Devices and is one of the corner stones of the MDR 2017/745 for Medical Devices and MDR 2017/746 for In Vitro Diagnostic Medical Devices.
The goal is to enhance the overall transparency for the sector and provide better access to information for the public and healthcare professionals. Behind the scenes, Notified Bodies and Competent Authorities will also make registration certificates which will improve market control.
A lot of the data manufacturers will have to provide in Eudamed will be publicly available, some information however will be restricted for Competent Authorities and Notified Bodies.
Eudamed will be composed of 6 interconnected modules and a public website. These modules are:
- Actor registration (active since 12/2020)
- UDI/Device registration (active since 10/2021)
- Notified Bodies and Certificates (partially active since 10/2021)
- Clinical Investigations and performance studies (under development)
- Vigilance and post-market surveillance (under development)
- Market Surveillance (under development)
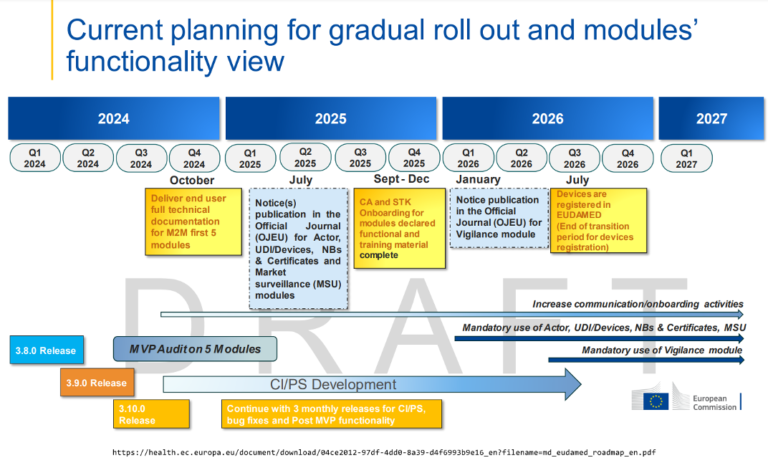
Back in 2017, with the publication of the MDR, the regulator had set out the requirements for Eudamed, which were very ambitious. Time has shown that the timeline was a bit too ambitious, as is the case with other aspects of the MDR. Originally, Eudamed would only become applicable once fully functional, but due to the magnitude and complexity of the project, this leads to a significant delay for Eudamed to become mandatory applicable, even though multiple modules are already operational.
This created additional workload for manufacturers and regulators, as manufacturers still need to register in individual countries, while the actor module and device module is already working. To take advantage of the modules that were already operational, and to reduce the workload for the stakeholders involved, a stepped approach was chosen where the finished modules would become mandatory before completion of all the modules.
On 9th July 2024, the amending Regulation (EU) 2024/1860 was published in the Official Journal of the European Union (OJEU) with immediate effect, amending the MDR regulations where the use of each module becomes mandatory, 6 months after it is declared functional following an independent audit, and the publication of a Commission notice to that effect in the Official Journal of the European Union. If all goes according to the plan, the transition plan for device registration ends in July 2026, and the use of the first modules will become mandatory.
As the mandatory use of Eudamed becomes closer, it’s very interesting for manufacturers to visit the playground of Eudamed to get familiarized with the different modules available, but also how and which information needs to be entered. Multiple videos are available from the European Commission in the Eudamed Information Center, demonstrating how to make your registrations.
Of course, the first step is to set up your account by registering as an actor in Eudamed. From then on, you will be able to register your UDI’s in the device module.
One of the problems our industry has is the large number of product variations. This led to the development of a Master UDI solution, which will be a grouping of UDI for similar products. The Master UDI will first become applicable for contact lenses but was developed for use in other highly individual devices as well. The delegated act EU Regulation 2023/2197 on Master UDI was published back in the summer of 2023, but the industry is still waiting on a guidance document on how to practically assign and implement this delegated act. Industry associations are working hard together with the European Commission to have this published in Q4 2024.
In the absence of the vigilance module, vigilance reporting needs to be done using the standardized MIR (Manufacturer’s Incident Report) template. This should be completed and submitted to your local Competent Authority. This system will of course be replaced, once the Vigilance Module of Eudamed becomes operational and mandatory.
Eudamed is also being prepared for Master UDI, so if you go to the playground of Eudamed, test version 3.9, you will be able to evaluate the current possibilities of Eudamed including Master UDI registration. There are still questions on how and what to enter in the different attribute fields for Master UDI, but industry associations are working hard together with the Eudamed team to have the necessary modifications and answers for the next release.
As often is the case with major ambitious projects, Eudamed has had his fair share of delays, but hopefully this will lead to a more efficient registration and monitoring system and ultimately a higher level of patient safety.


